Over the past few years, several areas in healthcare have seen unprecedented breakthroughs that promise to transform the healthcare landscape. Each doubles and triples down on the need to connect and curate the world’s healthcare to make it accessible and valuable. One such breakthrough we witness today is SaMD, or software as a medical device.
Software solutions are becoming increasingly important and pervasive in healthcare. Given the availability of a multitude of technology platforms (e.g., personal computers, smartphones, network servers, etc.), as well as increasing ease of access and distribution (e.g., internet, cloud), medical software development, for both medical purposes (software used to make clinical decisions) and non-medical purpose (e.g., administrative, financial) has seen a massive boom. Statista foresees that the medical device software market will yield an annual growth rate of 5.23% (CAGR 2024 – 2028).
What is SaMD?
SaMD is a medical instrument software “on its own” employed for medical purposes.
A SaMD generates evidence of the product’s analytical, technical, and clinical validation and proves a concrete clinical association between the output and the intended clinical condition. For instance, a healthcare software solution that collects a large amount of data from multiple sources (x-rays, scans, etc.) and transforms that data into 3D models can be seen as SaMD.
To simplify it with a use case, SaMD is software that allows a healthcare professional to view images from an MRI machine on a smartphone for diagnostic purposes. As per The International Medical Device Regulators Forum (IMDRF), SaMDs are built to respond to an algorithm that would produce a result or output based on input and reference data.
Software is crucial in telemedicine, remote monitoring, and AI-powered medical imaging analysis, most of which are built using general guidelines. However, In 2016, the International Medical Device Regulators Forum (IMDRF) proposed the first draft of SaMD regulation guidelines, which many countries later adopted. These guidelines ensure the safety, efficacy, and performance of SaMD while promoting innovation in the digital health industry.
As per the guidelines shared by FDA’s IMDRF, mentioned below is what can be “called” SaMD
- SaMD is the medical software that is compatible with medical devices.
- SaMD can run on general-purpose (non-medical purpose) computing platforms.
- “without being part of” means software is unnecessary for a hardware medical device to achieve its intended medical purpose.
- The software only meets the definition of SaMD if its intended purpose is to drive a hardware medical device.
- SaMD may be combined (e.g., as a module) with other products, including medical devices.
- SaMD can be interfaced with other medical devices, other SaMD software, and general-purpose software not intended for medical use
- Mobile apps that meet this definition above will be considered SaMD.
Examples of SaMD
Software as a medical device can be integrated with other medical devices, including hardware medical devices, other medical device software, and general-purpose software, such as treatment planning software that supplies information used in a linear accelerator.
There has been a rapid increase in the development of SaMD in recent years, and some examples of such devices are:
- Software intended to diagnose a condition using an accelerometer in a digital camera
- Software that allows MRI or other medical imaging to be viewed on mobile devices
- Software that performs image processing and detects stages of cancer
- Treatment planning applications that streamline information
- Software that controls an installed medical device
- BMI and body fat calculators and heart rate monitors
SaMDs vs. Non- SaMDs
| SaMD | Non-SaMD |
| Software that is connected to a hardware medical device but is not needed by that hardware medical device to achieve its intended medical purpose | Software that is required by a hardware medical device to perform the intended use, even if sold separately from the hardware medical device. |
| Software that detects and diagnoses a stroke by analyzing the image of an MRI | Software that records key administrative, clinical data relevant to that person’s care under a particular provider, like EHRs |
| Software that controls an installed medical device | Software used to “drive or control” the motors and the pumping of medication in an infusion pump. |
SaMD Regulation Around The World: Who’s Watching?
The FDA has released some guidelines relating to SaMD to help control the process of using this type of software in the field. The Software as a Medical Device Working Group (WG) — was formed in 2013 by the IMDRF and chaired by the FDA. However, the guidelines for the EU are similar to those of the US, except for the governing body.
| US | EU |
| In the US, you must follow the FDA’s Quality System Regulations
(QSR). |
In the EU, your SaMD will be governed by the EU MDR and GDPR (or EU IVDR if it is an in vitro diagnostic device). |
Here are the regulator agencies from different nations that ensure the quality control and analysis of SaMD development.
Japan: PMDA (Pharmaceuticals and Medical Devices Agency)
China: NMPA (National Medical Products Administration)
Australia: Data Protection Act, MHRA, Medicines & Healthcare products Regulatory Agency
India: CDSCO (Central Drugs Standard Control Organisation)
Canada: CMDR (Canadian Medical Device Regulations ), PIPEDA (Personal Information Protection and Electronic Documents Act)
Brazil: ANVISA (Brazilian Health Regulatory Agency)
Other than this, ISO 13485:2016, a globally recognized standard for quality management systems in the medical instrument industry, supports medical software development companies in designing quality management that establishes and maintains the effectiveness of their processes. This standard ensures the consistent design, development, production, installation, and delivery of health tech devices that are safe for their intended purpose. (view section, software architectural design).
SaMD Programming Model
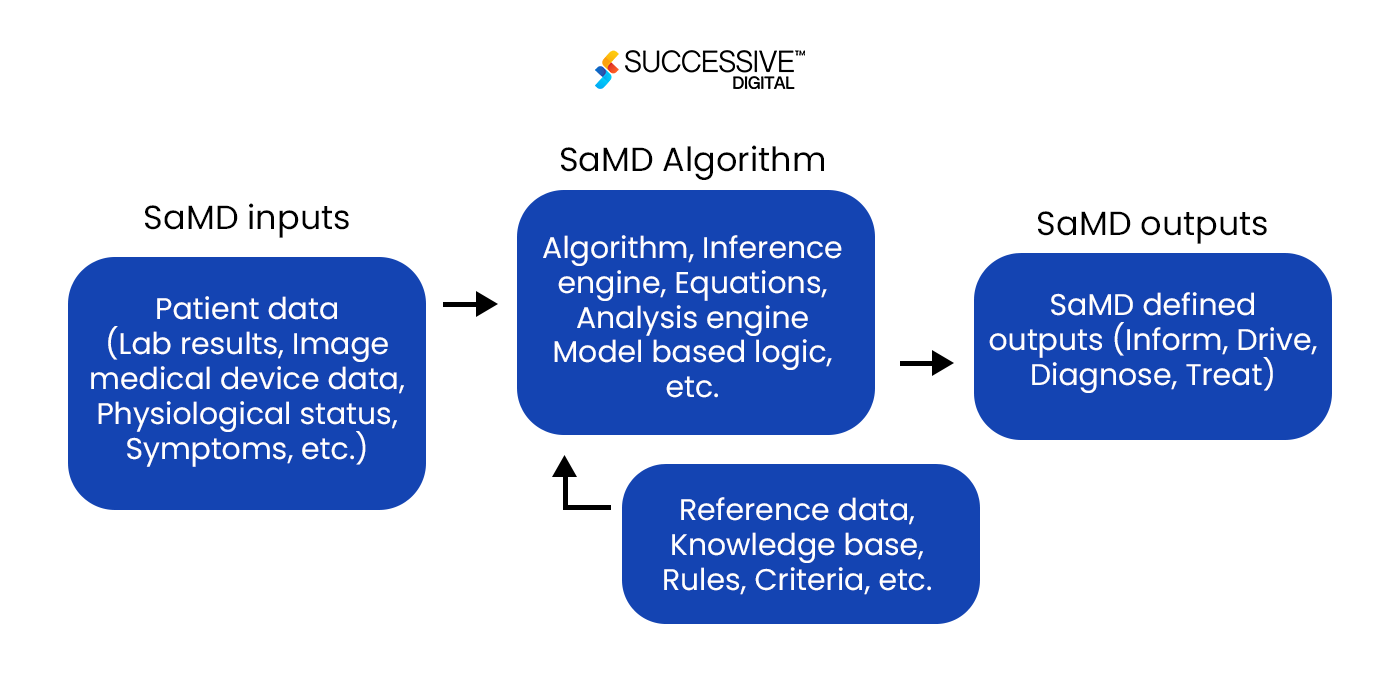
SaMD can run on smartphones, tablets, laptops, or other electronic devices at most user’s fingertips. Since SaMD doesn’t need a specific medical device to function, it can interface with various medical devices to carry out medical functions, ranging from diagnosis and prevention to monitoring and treating diseases and physiological conditions.
SaMD takes in various data inputs, like lab results, patient symptoms, or imaging data, and processes them using different algorithms. The software then produces outputs for multiple medical purposes, including helping users diagnose, treat, and manage their health issues.
Benefits of Software as a Medical Device
For Patients
With the help of SaMDs, Patients can play a more active role in managing their health. For instance, patients with asthma can use smart sensor data to identify and avoid triggers of flare-ups. Here are all the benefits of using SaMD for Patients
- Improved access to care at the fingertips
- Personalized care recommendations
- Real-time feedback, and support for personalized care
For Providers,
SaMD tools can assist clinicians in outperforming the desired medical diagnoses and detecting certain diseases earlier. For example, for healthcare companies under value-based care models, the Center for Medicare and Medicaid Innovation (CMMI) promotes prescribing the SaMD to patients to achieve positive health outcomes, reducing provider payment delays.
Here are other benefits of using SaMD for Providers
- Data accuracy and analysis
- Reduced errors
- Better medical device management
- Reduced medical costs
For Payers,
Software as a medical device (SaMD) can help payers improve the health and well-being of their members by providing personalized advice based on their health data and promoting preventive care and lifestyle changes that can contribute to better overall well-being. As SaMD promotes real-time results, regular monitoring and feedback can lead to early intervention, reducing the need for hospital visits and improving the quality of life for members with ongoing health concerns. Payers can also extend their services to remote or underserved members using SaMD for virtual consultations, remote diagnostics, and online healthcare services.
Here are other benefits of SaMD for payers.
- Improved outcomes for their members
- Preventative care through predictive analytics
- Personalized medicine to reduce care costs
- Telemedicine and Remote Patient Monitoring (RPM)
Software Development For SaMD As Per IEC 62304
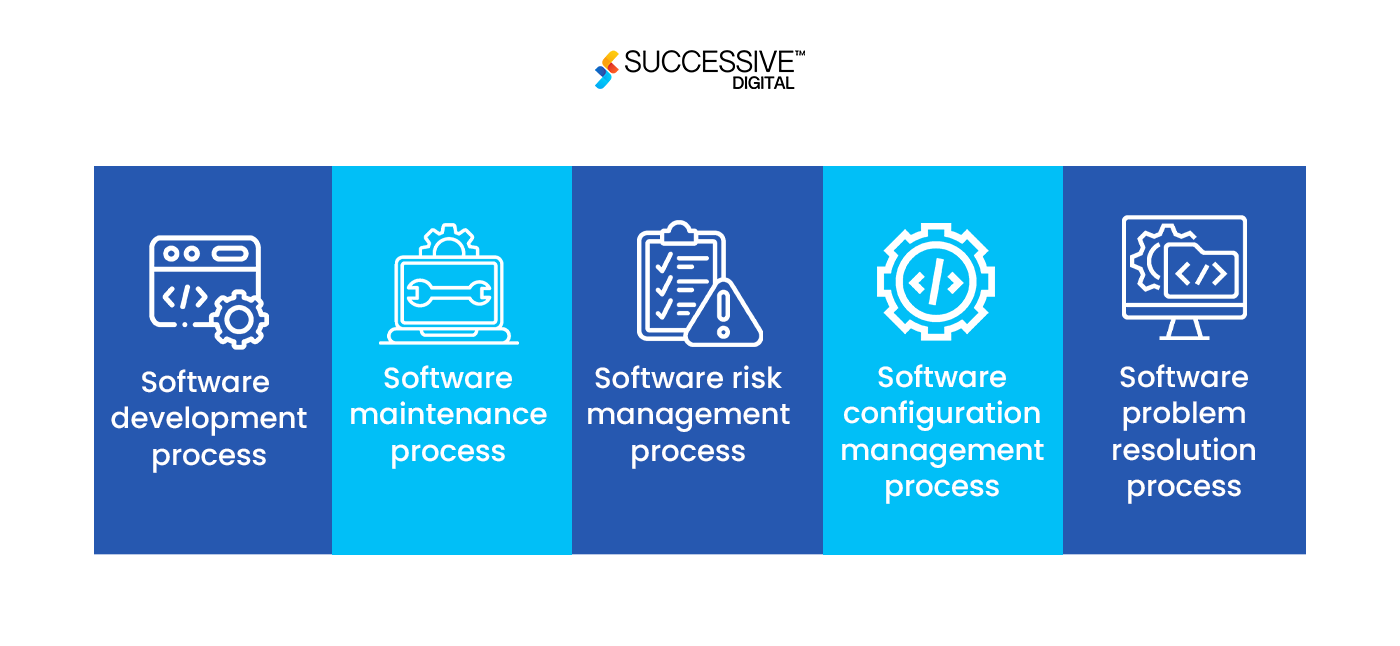
The IEC 62304, ISO regulatory practices for SaMD development, explains five processes to be implemented during the lifecycle of your software product development:
- Software development process
- Software maintenance process
- Software risk management process
- Software configuration management process
- Software problem resolution process
SOFTWARE DEVELOPMENT PROCESS
According to IEC 62304, the development process for SaMD begins with software development planning and ends with the software release.
The process includes:
-
Software requirements analysis
The SaMD manufacturer must gather all the necessary information for the general purpose SaMD and how it will be interfaced once released. Start by,
- Identifying the software concept.
- Prioritizing software requirements.
- Creating a detailed specification of the software.
SaMD development insists on adopting a risk management process compliant with ISO 14971 for risk analysis from the early development stage.
-
Software architectural design
Unlike any other software, architectural design in SaMD helps define performance, quality, scalability, maintainability, manageability, and usability.
Designing SaMD relies on and impacts the user experience to a great extent.
Consider, for example, a Glucose Monitor app for a smartwatch. The interface must be easy for a tech-savvy young adult to navigate but also simple enough for an older adult who may need to be more comfortable with technology. This delicate balance is one of the world’s most vital medical device software design challenges.
FDA current guidance (from 2005) divides SaMD design into three categories, known as “levels of concern,” which are based on the severity of injury that could arise from device failure or design flaws:
- Minor – failures or latent design flaws that are unlikely to cause any injury to the patient or operator.
- Moderate – failures or latent design flaws that could directly result in minor injury to the patient or operator, including through delayed or incorrect information or a provider’s actions.
- Major – failures or latent design flaws that could directly result in death or severe injury to the patient or provider, including through delayed or incorrect information or the actions of a provider.
-
Software unit implementation and verification:
The manufacturer of medical device software shall perform the software unit verification, and results shall be documented. Software unit verification means verifying the medical instrument software unit implementation against the software unit design.
-
Software integration and integration testing:
ISO’s IEC 62304 explains that SaMD shall be interoperable. Hence, software integration testing should focus on transferring data and control across a software module’s internal and external interfaces, such as those associated with medical device hardware, operating systems, and third-party software applications and libraries.
-
Software system testing:
The manufacturer collects real-time performance data to ensure continued safety and effectiveness once the product is introduced. This will help rectify errors and improve the device’s efficiency.
Also, to fulfill the guideline requirements of the FDA 510 (k) premarket notification and the Council of the European Union, SaMD shall comply according to ISO 13485, IEC 62304, and IEC 82304-1. This step is critical to ensure the software meets all regulatory requirements and is safe for use.
SOFTWARE MAINTENANCE PROCESS
According to the FDA, 79% of all errors occur during maintenance. Hence, to avoid these, the defined process models for maintenance outlined in IEC 62304 consist of three parts:
- Establishing your software maintenance plan
- Analysis of problems and modifications
- Implementing modifications
Software maintenance post-launch should be a part of the same phases as initial software development. The tests can be run as regression tests to ensure decreased bugs during the maintenance phase.
SOFTWARE RISK MANAGEMENT PROCESS
This process in the SaMD lifecycle ensures that errors are analyzed, trends are evaluated, and the appropriate measures (correction, reporting to authorities, etc.) are taken.
The risk management requirements in IEC 62304 correlate with those in ISO 14971, the international standard on the application of risk management to medical devices, which sums up as,
- An analysis of software that contributes to hazardous situations
- Risk control measures
- Verification of risk control measures
- Risk management of software changes
The development process in software as a medical device can be used as a risk control measure when it’s carried out with adherence to IEC 62304.
SOFTWARE CONFIGURATION MANAGEMENT PROCESS
Carrying out the configuration management process is most crucial in SaMD development as it facilitates orderly management of product information and changes for purposes such as revising capability, improving performance, reliability, or maintainability, extending life, reducing cost, reducing risk and liability or correcting defects as and when they occur.
SOFTWARE PROBLEM RESOLUTION PROCESS
The final section for the development of SaMD under IEC 62304 procedures explains the processes that have been used to resolve software problems and outlines how other issues will be tracked and evaluated if they arise in the future. The procedure outlines the preparation of problem reports, investigation of the problems, control process, analysis of problems for trends, and verification of software problem resolution.
Recent Developments and Opportunities for SaMD
As medical devices evolve into smart, connected, and scalable solutions over the years, they enhance the healthcare experience across the trifecta value chain – for patients, care providers, and insurance companies. Recent developments across technologies like cloud, mobility, extended reality, and artificial intelligence/machine learning are upending the conventional solution approaches of development for Software as a Medical Device (SaMD).
Telemedicine: Years of exploration caused this developing innovation of telemedicine to become more widely adopted in 2020, primarily due to the COVID-19 pandemic. RPM and telemedicine are critical pieces of technology that allow care to be delivered at a distance. Software product development companies can build SaMD to facilitate remote consultations, monitor patient health data, and provide real-time feedback to healthcare providers.
Value-Based Care: ISO’s regulated SaMD promotes fair data practices and protocols for exchanging and using data in transit and data at rest, giving greater visibility in identifying the care gaps, a huge challenge within the healthcare ecosystem across the globe. Hence, the SaMD manufacturers can develop more interoperable software solutions to help medical companies hit their metric goals for medical device integration.
Artificial Intelligence and Machine Learning: In some cases, manufacturers build SaMD to support in-patient and out-patient cases through data and clinical efficiency management, where patient data transparency and security are paramount. This involves sensor data acquisition, connectivity, cloud integration, and user application development. SaMDs can leverage AI/ML technologies to enhance the accuracy of diagnostic (imaging and non-imaging), prescriptive use cases, and personalized treatment solutions.
Wearables and IoT devices: The growth of IoT or IoMT (Internet of Medical Things, a connected infrastructure of medical tools, software applications, and health systems) has opened opportunities for SaMD companies to develop interoperable and connected devices with the growing graph of IoT/IoMT use cases allow devices to communicate and exchange data, improving efficiency and patient care and providing real-time monitoring for health metrics.
Successive’s View
Software as a Medical Device has emerged as a powerful tool to help hospitals and health systems effectively empower providers to enhance care quality and provide payer-agnostic data to inform clinical, quality, and risk adjustment programs to improve patient care outcomes.
About 30 percent of healthcare resources are wasted every year due to the absence of accurate data. However, when records and data are correctly organized and able to be analyzed, this helps providers create a better and more efficient system designed to deliver honest and helpful healthcare to their patients and be financially accessible for the providers.
SaMD is used widely for medical purposes, such as diagnosing, monitoring, treating, or preventing diseases and improving cost-effective care outcomes.
By promoting personalized health, SaMDs allow for more guided care that addresses social determinants of health (SDoH)—socioeconomic factors that affect and result in more proactive “upstream” healthcare to prevent health problems from developing.
How Can We Help?
Over the last decade, Successive has built over 100+ healthcare software solutions interoperable with medical devices, intending to enhance care.
Our software developers possess the prowess to build FDA-compliant healthcare solutions alongside technical acumen.
Recently, we have contributed to the medical software development of a US-based integrated virtual care solution. With primary care, behavioral health, and urgent care being their core offering, we helped the organization build a solution with a holistic suite of virtual care services and supplemental benefits for users to design a digital health solution.
The software has the power to improve the diagnosis and treatment of various diseases and enable patients to manage their health better. As a healthcare software development company, we contribute by building scalable, technically poised, and compliant solutions.
Explore More
We have compiled an exclusive guide for HIPAA Compliant App Development for the Healthcare Industry to understand healthcare compliance better.
Browse the guide here.